Acids and Bases
Acids and bases are everywhere, from the sour deliciousness of lemon to the cleaning power of soap. This idea helps explain what allows a substance to be an acid or base. How to test acids and bases. And how acids and bases can be useful in everyday life! With detailed explanations, fun examples, and simple activities, this guide on acids and Bases with Real-Life examples will provide you with a thorough conceptualization of the idea.
Table of Contents
- What Are Acids?
- What Are Bases?
- Know About Neutralization Reaction
- Salts: Formation, Nature and Type
- The pH Scale – Measuring Acidity and Basicity
- Indicators: Types and Visual Demonstrations
- Key Differences Between Acids and Bases
- Applications and Uses in Daily Life
- Tips and Tricks to Learn the Concepts Easily
- Conclusion
- FAQs
What Are Acids?
An acid is a compound that, when you place it in water, releases hydrogen ions, or H⁺. The hydrogen ion is what gives acid its sour flavor as well as the capacity to react vigorously with other materials. Acids are not just something you read about in a textbook; they are all around you.
They can be found in the fruits you eat, the cleaning solutions you may use, and in your stomach to break down food when digestion occurs.
Some Common examples are:
HCl→H++Cl−
H2SO4→2H++SO42−
Properties of Acids
-
They taste sour. For example, lemon juice and tamarind have that sharp, tangy flavor due to the acids they contain.
-
They turn blue litmus paper red, which is a common way to test for acids in the lab.
-
They conduct electricity when dissolved in water, which means they allow an electric current to pass through.
-
Strong acids can be corrosive, meaning they can wear away metals or skin if not handled properly.
-
Acids react with certain metals to release hydrogen gas, often seen as bubbling or fizzing during the reaction.
Real-Life Examples of Acids
|
Acid |
Common Source |
Use |
|
Citric acid |
Citrus fruits (lemon, lime) |
Adds sour flavor, used in cooking |
|
Acetic acid |
Vinegar |
Used in cooking and pickling |
|
Hydrochloric acid |
Stomach |
Helps digest food |
|
Carbonic acid |
Soft drinks |
Provides fizz |
What Are Bases?
Bases are substances that donate hydroxide ions when dissolved in water. The presence of hydroxide ions gives bases their familiar bitter taste and slippery feel. You likely encounter bases more often than you think. Bases can be found in soaps, toothpaste, cleaning products, and even in some medicines that help mitigate stomach acid.
Some Common examples are:
NaOH→Na++OH−
KOH→K++OH−
Properties of Bases
-
Bases have a bitter taste. For example, baking soda has a slightly bitter taste.
-
Bases feel slippery or soapy to the touch, like hand wash or liquid soap.
-
Bases change red litmus paper blue, which is how we commonly test for bases in the laboratory.
-
Bases can carry an electric current by conducting electricity when dissolved in water.
-
Bases react with acids in a reaction that forms a salt and water, called neutralization.
Real-Life Examples of Bases
|
Base |
Common Source |
Use |
|
Sodium hydroxide |
Soap, cleaners |
Used in drain cleaners |
|
Magnesium hydroxide |
Antacid tablets |
Used to treat indigestion |
|
Ammonium hydroxide |
Household cleaning liquid |
Used for surface cleaning |
|
Calcium hydroxide |
Limewater |
Used in whitewashing walls |
Know About Neutralisation Reaction
When an acid and a base react, they cancel each other’s properties and form a salt and water. This is called a neutralization reaction.
Let's Take an Example:
Hydrochloric acid + Sodium hydroxide → Sodium chloride (salt) + Water
Neutralisation in Daily Life
-
Indigestion Relief: Magnesium hydroxide (milk of magnesia) neutralises excess acid in the stomach.
-
Insect Stings: Formic acid from stings is neutralised with baking soda.
-
Soil pH Balance: Lime (a base) is added to acidic soil for farming.
-
Waste Management: Factories use bases to neutralise acidic waste before release.
Salts: Formation, Nature and Types
Salts are chemical compounds formed when acids react with bases. This reaction is called neutralisation.
For example: HCl + NaOH → NaCl + H₂O
Sodium chloride (NaCl) is the salt produced in this case. Salts may be:
-
Neutral: e.g., sodium chloride
-
Acidic: e.g., ammonium chloride
-
Basic: e.g., sodium carbonate
Salts are used in cooking, agriculture, manufacturing, and chemical processes.
To clearly understand whether a solution is acidic or basic, we rely on special tools called indicators. Let’s explore how indicators work and the types used in science.
The pH Scale :Measuring Acidity and Basicity
The pH scale tells us how acidic or basic a solution is. It ranges from 0 to 14:
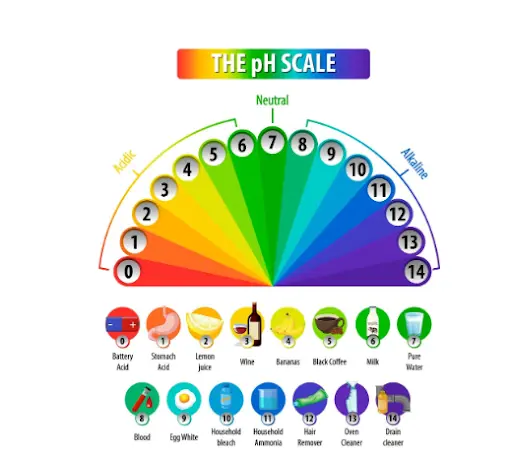
-
0–6: Acidic (like lemon juice or vinegar)
-
7: Neutral (like pure water)
-
8–14: Basic/Alkaline (like baking soda or soap)
If the value is lower, it is a stronger acid; the higher the number, the stronger the base. The strength increases or decreases by a factor of 10 between each number. The pH scale is simple and powerful, and takes about one second to identify what we are working with sour or slippery.
Indicators: Types and Visual Demonstrations
Indicators can help us test to see if certain substances are acidic or basic. The following table provides an overview of different types of indicators and how they behave:
-
Natural Indicators: Found in nature, change colours in acids and bases.
-
Synthetic Indicators: Man-made Indicators, such as phenolphthalein or methyl orange, when used in laboratories, can help provide accurate detection of pH levels.
-
Olfactory Indicators: Change their smell in acidic or basic solutions.
-
Visual Indicators: Indicate a visible colour change when detecting acids or bases, making it easy to see.
| S.No. | Indicator | Smell/Colour in Acid Solution | Smell/Colour in Basic Solution |
|
Natural Indicators |
|||
|
1 |
Litmus |
Red |
Blue |
|
2 |
Red cabbage leaf extract |
Red |
Green |
|
3 |
Flowers of hydrangea plant |
Blue |
Pink |
|
4 |
Turmeric |
No change |
Red |
|
Synthetic Indicators |
|||
|
1 |
Phenolphthalein |
Colourless |
Pink |
|
2 |
Methyl orange |
Red |
Yellow |
|
Olfactory Indicators |
|||
|
1 |
Onion |
Characteristic smell |
No smell |
|
2 |
Vanilla essence |
Retains smell |
No smell |
|
3 |
Clove oil |
Retains smell |
Loses smell |
Key Differences Between Acids and Bases
Acids and bases are opposites in nature one’s sour and sharp, the other’s bitter and slippery. Let’s spot the key differences.
|
Characteristic |
Acids |
Bases |
|
Taste |
Sour |
Bitter |
|
Feel |
Not slippery |
Slippery |
|
Litmus Test |
Turns blue litmus red |
Turns red litmus blue |
|
pH Value |
Less than 7 |
Greater than 7 |
|
Reaction |
React with bases |
React with acids |
Applications and Uses in Daily Life
-
We use acids in food preservation, baking, and cooking.
-
Bases are used to make soaps, antacids, and cleaners.
-
Salts are found in table salt, fertilizers, and in many of the chemicals we use.
-
Fire extinguishers contain compounds like aluminium hydroxide, which allow for foaming.
Tips and Tricks to Learn the Concepts Easily
Learning acids and bases can be fun and easy when we link the concepts with everyday living.
-
Litmus fun – Acids turn blue litmus red, and bases red litmus blue. Put this tip near your study desk!
-
Learn from what you see around – Vinegar in your kitchen is an acid, and soap in your bathroom is a base.
-
Act like you are a scientist in real life – Use vinegar (acid) in the kitchen and soap (base) in the bathroom.
-
The pH Trick - pH < 7 = Acid, pH > 7 = Base. Draw a colourful scale to help remember.
Conclusion
Acids and bases are an integral part of our everyday lives; from the food we eat to the cleaners we use, they affect almost everything in our daily lives. By understanding how they work, we can appreciate everyday activities such as cooking, digestion, and even health. When students learn how to test and use them safely, they are involved in science. Be curious; there is science everywhere, and wherever there is science, the fun is extremely easy to find!
FAQs
Q1: What is salt in chemistry?
Answers:Salt is a compound produced from the reaction of an acid with a base.
Q2: What do indicators do?
Answers:Indicators identify whether a solution is acidic or basic through a change in colour, or smell.
Q3: What is phenolphthalein used for?
Answers:It is used as an indicator in acid-base reactions, and it turns pink in bases or basic solutions.
Q4: What happens if the soil is too acidic?
Answers:Farmers apply substances to the soil that are basic, like lime, to neutralise the acidic soil for more productive crops.
Q5: Are all salts neutral?
Answers:No. Some salts are either acidic or basic, depending on whether the acid and base are stronger or weaker.
Q6: Can you give some examples of acids and bases?
Answers:Examples of acids and bases are: Citric acid (lemon), Acetic acid (vinegar), Sodium hydroxide (soap), and Calcium hydroxide (limewater).
Q7: What is the difference between acids and bases?
Answers:The biggest difference between acids and bases is that acids have a pH of less than 7, blue litmus turns red, and bases have a pH greater than 7, red litmus turns blue.
CBSE Schools In Popular Cities
- CBSE Schools in Bangalore
- CBSE Schools in Mumbai
- CBSE Schools in Pune
- CBSE Schools in Hyderabad
- CBSE Schools in Chennai
- CBSE Schools in Gurgaon
- CBSE Schools in Kolkata
- CBSE Schools in Indore
- CBSE Schools in Sonipat
- CBSE Schools in Delhi
- CBSE Schools in Rohtak
- CBSE Schools in Bhopal
- CBSE Schools in Aurangabad
- CBSE Schools in Jabalpur
- CBSE Schools in Jaipur
- CBSE Schools in Jodhpur
- CBSE Schools in Nagpur
- CBSE Schools in Ahmednagar
- CBSE School In Tumkur











