Understanding the Theories of Acids and Bases
Theories of Acids and Bases-Ever wondered what makes lemon juice acidic or soap feel slippery? From the tangy taste of a lemon to the slippery feel of soap, these interactions are all about acids and bases at work. Over time, scientists have developed different theories to explain what makes a substance an acid or a base, because a single definition could not capture all their behaviors.
This articles covers from the classic Arrhenius definition to the broader Lewis theory, you’ll learn how scientists have explained acid-base behavior over time.
Table of Contents
- What are acids and bases?
- Theories Based on the Concepts of Acids and Bases
- Tips and Tricks to Learn the Concepts Easily
- Conclusion
- FAQs
Theories Based on the Concepts of Acids and Bases
What are Acids and Bases?
Acids and bases are fundamental concepts in chemistry that are essential to understanding the nature of many chemical reactions and the environment in which they occur.
Generally, we define acids as substances that contain hydrogen, which can donate Hydrogen ions, and bases as compounds that display hydroxide ions, which can accept them in a solution.
Arrhenius's Theory of Acid and Base
In the 1880s, the Swedish scientist Svante Arrhenius published a theory that focused only on reactions that take place in water. According to Arrhenius
-
Acids are substances that produce hydrogen ions (H+) in aqueous solutions.
Examples of acids include: Hydrochloric acid (HCl) and sulphuric acid (H2SO4).
HCl(aq)→H+(aq)+Cl−(aq)
H2SO4(aq)→2H+(aq)+SO42−(aq)
-
Bases are substances that produce hydroxide ions (OH-) in aqueous solutions.
Examples of bases include: Sodium hydroxide (NaOH) and potassium hydroxide (KOH).
NaOH(aq)→Na+(aq)+OH−(aq)
KOH(aq)→K+(aq)+OH−(aq)
Bronsted-Lowry Acid-Base Theory
To build on the work of Arrhenius, scientists Johannes Bronsted and Thomas Lowry expanded the Acid-Base theory in 1923. They stated that:
-
Acids are proton (H⁺) donors.
-
Bases are proton (H⁺) acceptors.
Their theory encompassed acid-base behavior in both aqueous and non-aqueous solutions, making it a broader concept.
In addition to the ability to donate and accept protons, they presented the idea of conjugate acid-base pairs, characterizing the acid and the conjugate base or the base and the conjugate acid:
-
Example:
[CH3COOH+H2O⇌CH3COO−+H3O+][Acid:CH3COOH,Conjugate Base: CH3COO−][Base: H2O,Conjugate Acid: H3O+]
Some substances can act as both an acid and a base in different reactions, referred to as an amphoteric substance.
Lewis Acid-Base Theory
In 1923, Gilbert N. Lewis introduced a different view of acid-base interactivity and simply focused on the interactions of electron pairs:
-
Lewis acids are electron pair acceptors
-
Lewis bases are electron pair donors
Lewis acid-base concepts can explain acid-base reactions that do not involve hydrogen ions, thereby providing a more comprehensive understanding of the broader area of acid-base behavior.
This also allows many more types of substances and reactions to be interpreted by acid-base behavior.
-
Example: H++OH⁻→H₂O
H⁺ (hydrogen ion) acts as the Lewis acid because it accepts an electron pair. OH⁻ (hydroxide ion) acts as the Lewis base because it donates an electron pair to form water.
Acid-Base Indicator Theory
These are substances that indicate acidity or basicity in a solution, usually with a color change. They help, for instance, in titrations as well as pH measurement.
In Ostwald's ionization theory, the indicator, such as phenolphthalein, is a weak acid or a weak base itself. Its color depends on the ionization and the pH of the solution:
Phenolphthalein is colorless in weakly basic solutions, and pink in strongly basic solutions (pH 8.0–9.8).
Quinonoid Theory
This theory explains that acid-base indicators are also organic aromatic compounds in equilibrium between two forms:
-
Benzenoid form: usually lighter in color
-
Quinoid form: usually darker in color
The color change that you observe while titrating a solution that is changing pH is due to the indicator transitioning between the two forms.
Tips and Tricks to Learn the Concepts Easily
-
Think simple mnemonics: A for Acid (H⁺ donor), B for Base (OH⁻ donor).
-
Relate to real life: Connect acids with lemon and vinegar; bases with baking soda and soap.
-
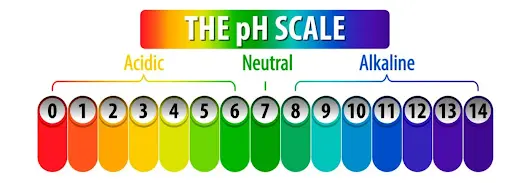
Sketch quick visuals: A colorful pH scale or acid-base reactions can boost memory.
-
Break it into theory groups: Make small tables comparing Arrhenius, Bronsted-Lowry, and Lewis.
-
Test yourself regularly: Use flashcards or short quizzes to revise key definitions and examples.
Conclusion
Each acid-base theory provides a unique framework for understanding chemical reactions. From the early Arrhenius model to the Lewis theory, these theories collectively enhance our comprehension of acid-base chemistry. Their applications extend beyond laboratories into medicine, agriculture, and household products.
FAQs
1. What are the main theories that define acids and bases?
Answers:The three key models are:
-
Arrhenius: Defines acids as H⁺ producers and bases as OH⁻ producers in water.
-
Brønsted–Lowry: Acids donate protons (H⁺), and bases accept them.
-
Lewis: Acids accept electron pairs, and bases donate them.
2. How do Brønsted–Lowry and Arrhenius theories differ?
Answers: Arrhenius explains acid-base behavior only in water. Brønsted–Lowry expands on this by focusing on proton transfer, so it works in both aqueous and non-aqueous solutions.
3. Why is the Lewis theory more general than the others?
Answers:Lewis's theory isn’t limited to protons; it defines acids and bases based on electron pair exchange, allowing a wider range of reactions to be explained.
4. What is a conjugate acid-base pair?
Answers:It’s a pair formed when an acid gives up a proton and becomes its conjugate base or a base accepts a proton and becomes its conjugate acid, like H₂O/H₃O⁺ or NH₃/NH₄⁺.
5. Can a substance act both as an acid and a base?
Answers:Yes, substances like water can act as either, depending on the situation. These are called amphoteric substances.
6. What are the Arrhenius Concepts of Acids and Bases?
Answers:The Arrhenius Concepts of Acids and Bases state that acids are substances that increase hydrogen ion (H⁺) concentration in water, while bases increase hydroxide ion (OH⁻) concentration in water.
7. What does the Arrhenius Theory of Acids and Bases explain?
Answers:The Arrhenius Theory of Acid and Base explains acid-base behavior in aqueous solutions only. It focuses on ion production acids release H⁺ ions and bases release OH⁻ ions when dissolved in water.
8. How does the Bronsted-Lowry Acid-Base Theory define acids and bases?
Answers:The Bronsted-Lowry Acid-Base Theory defines acids as proton (H⁺) donors and bases as proton (H⁺) acceptors. This theory applies in both aqueous and non-aqueous environments.
9. What is the purpose of Acid-Base Indicator Theory?
Answers:The Acid-Base Indicator Theory describes how indicators show the acidic or basic nature of a solution through color changes. Indicators like litmus or phenolphthalein help visually determine pH levels.
10. What are the different Theories Based on the Concepts of Acids and Bases?
Answers:The Theories Based on the Concepts of Acids and Bases include the Arrhenius, Bronsted-Lowry, and Lewis theories. Each offers a different approach to classifying and understanding acid-base behaviour.
CBSE Schools In Popular Cities
- CBSE Schools in Bangalore
- CBSE Schools in Mumbai
- CBSE Schools in Pune
- CBSE Schools in Hyderabad
- CBSE Schools in Chennai
- CBSE Schools in Gurgaon
- CBSE Schools in Kolkata
- CBSE Schools in Indore
- CBSE Schools in Sonipat
- CBSE Schools in Delhi
- CBSE Schools in Rohtak
- CBSE Schools in Bhopal
- CBSE Schools in Aurangabad
- CBSE Schools in Jabalpur
- CBSE Schools in Jaipur
- CBSE Schools in Jodhpur
- CBSE Schools in Nagpur
- CBSE Schools in Ahmednagar
- CBSE School In Tumkur











