Solids, liquids, and gases
Everything around us is made up of matter, which exists in different forms. Let's explore the three primary states of matter: solids, liquids, and gases!
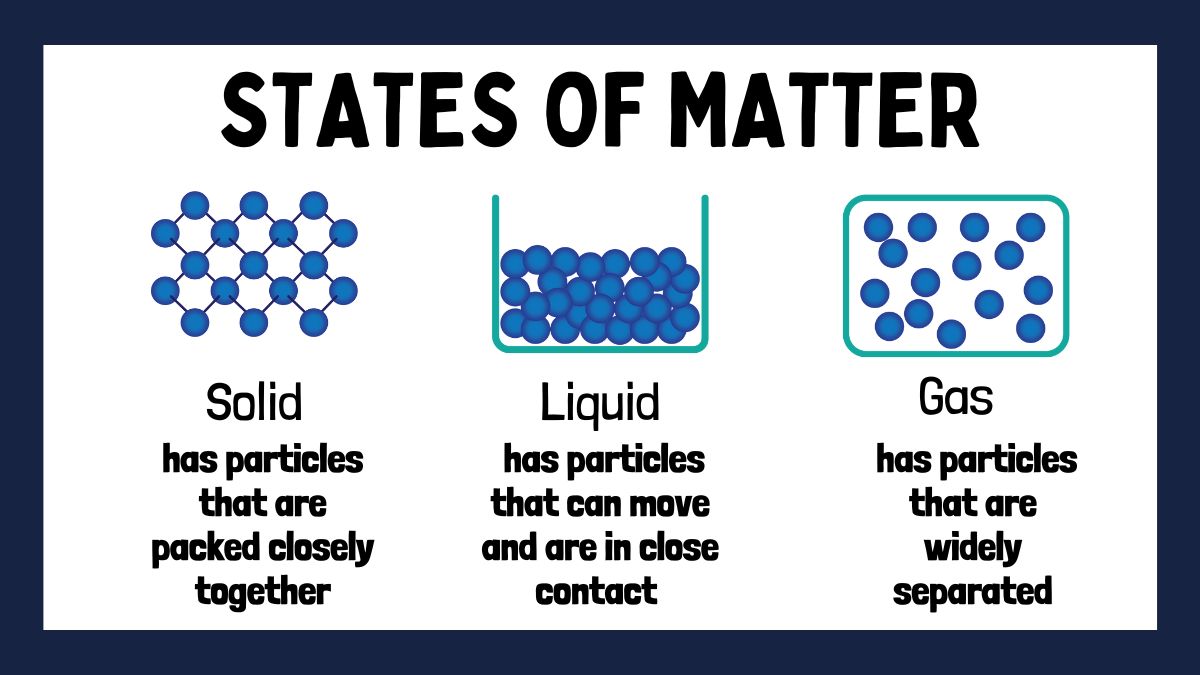
What Are States of Matter?
Matter exists in three main states:
Solids: The Strong and Steady
Solids have a fixed shape and volume. The particles in a solid are tightly packed and can only vibrate in place. This makes solids firm and steady.
Examples: rocks, pencils, toys, ice.
Fun Fact: Ice is solid. When it melts, it turns into liquid water!
Liquids: The Flowing Matter
Liquids have a fixed volume but take the shape of their container. The particles are close together but can move past each other, allowing liquids to flow.
Examples: water, milk, juice, oil.
Fun Fact: Water is pourable because its particles can shuffle around, but not as freely as gas!
Gases: The Wild and Free
Gases have no fixed shape or volume. The particles are far apart and move freely and quickly in all directions.
Examples: air, steam, oxygen, helium.
Fun Fact: Hot air balloons rise because hot air is lighter than the cooler air outside!
Changes Between States: The Magic of Matter!
Matter can change from one state to another through heating or cooling.
-
Melting: Solid to liquid (e.g., ice to water).
-
Freezing: Liquid to solid (e.g., water to ice).
-
Evaporation: Liquid to gas (e.g., water to steam).
-
Condensation: Gas to liquid (e.g., steam to water).
-
Sublimation: Solid to gas without becoming liquid (e.g., dry ice).
States of Matter and Examples:
1. Solids:
-
-
Examples: Ice, wood, metal, stone, and diamond.
-
In solids, particles are closely packed and vibrate in place, giving solids a fixed shape and volume.
2. Liquids:
-
-
Examples: Water, oil, maple syrup, and juice.
-
In liquids, particles are close together but can move past one another, giving liquids a fixed volume but no fixed shape (they take the shape of their container).
3. Gases:
-
-
Examples: Oxygen, nitrogen, carbon dioxide, and helium.
-
In gases, particles are spread out and move freely, allowing gases to expand and fill the shape and volume of their container
Differences Between Solids, Liquids, and Gases:
-
Shape:
-
Solid: Has a fixed shape.
-
Liquid: Takes the shape of its container.
-
Gas: Expands to fill the shape of its container.
-
Volume:
-
Solid: Has a fixed volume.
-
Liquid: Has a fixed volume but no fixed shape.
-
Gas: Has no fixed volume, expands to fill the entire volume of its container.
-
Particle Movement:
-
Solid: Particles vibrate in place, held tightly together.
-
Liquid: Particles can move around each other, but stay close.
-
Gas: Particles move freely and spread out.
-
Density:
-
Solid: High density (particles are tightly packed).
-
Liquid: Lower density than solids (particles are a bit further apart).
-
Gas: Very low density (particles are far apart).
-
Compressibility:
-
Solid: Cannot be compressed (particles are already tightly packed).
-
Liquid: Slightly compressible (particles can be pushed a bit closer).
-
Gas: Easily compressible (particles can be pushed much closer together).
Changes Between States: The Magic of Matter!
Matter can change state when heat energy is either absorbed or released. When you apply heat to a solid, it can undergo melting into a liquid (e.g., ice becomes water). On the other hand, If you cool a liquid, it may turn into a solid (for instance, when water becomes ice). The heating of a liquid allows it to escape as a gas (water becoming steam). These transformations are referred to as phase changes.
Fun Fact: Steam is water, but you have to heat it up. But if you chill the steam, it will revert to water. Isn’t that cool?
States of Matter and Examples:
-
Solids:
-
Examples: Ice, wood, metal, stone, and diamond.
-
In solids, particles are closely packed and vibrate in place, giving solids a fixed shape and volume.

-
Liquids:
-
Examples: Water, oil, maple syrup, and juice.
-
In liquids, particles are close together but can move past one another, giving liquids a fixed volume but no fixed shape (they take the shape of their container).

-
Gases:
-
Examples: Oxygen, nitrogen, carbon dioxide, and helium.
-
In gases, particles are spread out and move freely, allowing gases to expand and fill the shape and volume of their container.
Differences Between Solids, Liquids, and Gases:
-
Shape:
- Solid: Has a fixed shape.
- Liquid: Takes the shape of its container.
- Gas: Expands to fill the shape of its container.
-
Volume:
- Solid: Has a fixed volume.
- Liquid: Has a fixed volume but no fixed shape.
- Gas: Has no fixed volume, expands to fill the entire volume of its container.
-
Particle Movement:
- Solid: Particles vibrate in place, held tightly together.
- Liquid: Particles can move around each other, but stay close.
- Gas: Particles move freely and spread out.
-
Density:
- Solid: High density (particles are tightly packed).
- Liquid: Lower density than solids (particles are a bit further apart).
- Gas: Very low density (particles are far apart).
-
Compressibility:
- Solid: Cannot be compressed (particles are already tightly packed).
- Liquid: Slightly compressible (particles can be pushed a bit closer).
- Gas: Easily compressible (particles can be pushed much closer together).
Recap Time:
We’ve learned so much about solids, liquids and gases! Here’s a quick review:
-
Solids: definite shape and volume tight particle arrangement
-
Liquids: Definite volume, conforms to the shape of the container, particles slide past one another.
-
Gases: No distinct shape or volume, particles widely dispersed and in motion.
Conclusion
-
Solids are strong and steady with particles packed tightly.
-
Liquids can flow and take the shape of their container.
-
Gases are wild, fast, and spread in all directions.
-
Matter can change state through heating or cooling.
MCQs
-
What state of matter is toothpaste?
-
What happens when you boil water?
-
Why does a balloon pop when filled too much?
-
What state is steam?
CBSE Schools In Popular Cities
- CBSE Schools in Bangalore
- CBSE Schools in Mumbai
- CBSE Schools in Pune
- CBSE Schools in Hyderabad
- CBSE Schools in Chennai
- CBSE Schools in Gurgaon
- CBSE Schools in Kolkata
- CBSE Schools in Indore
- CBSE Schools in Sonipat
- CBSE Schools in Delhi
- CBSE Schools in Rohtak
- CBSE Schools in Bhopal
- CBSE Schools in Aurangabad
- CBSE Schools in Jabalpur
- CBSE Schools in Jaipur
- CBSE Schools in Jodhpur
- CBSE Schools in Nagpur
- CBSE Schools in Ahmednagar
- CBSE School In Tumkur











