(a) Let a drop of a liquid L be poured on a solid surface S placed in air A. If TSL,and TSA be the surface tensions corresponding to solid-liquid layer, liquid-air layer and solid-air layer respectively and θ be the angle of contact between the liquid and solid, then
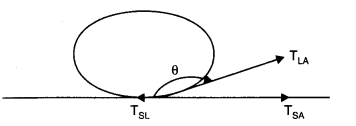
TLA Cos θ + TSL =TSA
=>Cos θ=TSA-TSL/TLA
For the mercury-glass interface, TSA< TSL. Therefore, cos 0 is negative. Thus θ is an obtuse angle. For the water-glass interface, TSA > TSL. Therefore cos 0 is positive. Thus, θ is an acute angle.
(b) Water on a clean glass surface tends to spread out i.e., water wets glass because the force of cohesion of water is much less than the force of adhesion due to glass. In case of mercury, cohesion due to mercury molecules is quite strong as compared to adhesion force due to glass. Consequently, mercury does not wet glass and tends to form drops.
(c) Surface tension of liquid is the force acting per unit length on a line drawn tangentially to the liquid surface at rest. Since h as force is independent of the area of liquid surface therefore, surface tension is also independent of the area of the liquid surface.
(d) We know that the clothes have narrow pores or spaces which act as capillaries. Also, we know that the rise of liquid in a capillary tube is directly proportional to cosθ (Here θ is the angle of contact). As θ is small for detergent, therefore cos θ will be large. Due to this, the detergent will penetrate more in the narrow pores of the clothes.
(e) We know that any system tends to remain in a state of minimum energy. In the absence of any external force for a given volume of liquid its surface area and consequently. Surface energy is least for a spherical shape. It is due to this reason that a liquid drop, in the absence of an external force, is spherical in shape.











